-
1. Solid State
11-
Crystalline & Amorphous Solid 50 minLecture1.1
-
Law of Crystallography 01 hourLecture1.2
-
Bravius lattice & Important Terms of solid state 48 minLecture1.3
-
Type of Cubic crystal & Closest packed St. 01 hourLecture1.4
-
Tetrahedral & Octahedral Void 38 minLecture1.5
-
Type of Voids & Radius Ratio 44 minLecture1.6
-
Type of ionic solid 59 minLecture1.7
-
Defect in Solid 48 minLecture1.8
-
Metallic Bonding 52 minLecture1.9
-
Chapter Notes – Solid StateLecture1.10
-
NCERT Solutions – Solid StateLecture1.11
-
-
2. Solution and its C.P
9-
Condition of solution formation, TD of Solution, Factors affecting solubility-Henary’s Law 55 minLecture2.1
-
Colligative Properties, Raoult’s Law 49 minLecture2.2
-
Relative lowering of V.P. & Problems 45 minLecture2.3
-
Non ideal solution, Azeotropic Solution 46 minLecture2.4
-
Elevation in B.P., Depression in F.P. 47 minLecture2.5
-
Osmotic Pressure, Abnormal C.P. & Van’t Hoff Factor 59 minLecture2.6
-
Solution – Ostwald Walker Exp. 13 minLecture2.7
-
Chapter Notes – Solution and its C.PLecture2.8
-
NCERT Solutions – Solution and its C.PLecture2.9
-
-
3. Chemical Kinetics
10-
Rate of reaction 37 minLecture3.1
-
Differential Rate Law 38 minLecture3.2
-
Integrated Rate Law 56 minLecture3.3
-
Integrated Rate problems 53 minLecture3.4
-
Pseudo order Reaction 40 minLecture3.5
-
Reaction Mechanism 47 minLecture3.6
-
Collision Model 34 minLecture3.7
-
Arhenius Equation 34 minLecture3.8
-
Chapter Notes – Chemical KineticsLecture3.9
-
NCERT Solutions – Chemical KineticsLecture3.10
-
-
4. Electrochemistry
13-
Introduction & Galvanic cell 32 minLecture4.1
-
Cell Notation & Cell Reaction 35 minLecture4.2
-
Electrode & Cell Potential 38 minLecture4.3
-
Electrochemical series 39 minLecture4.4
-
The Nernst Equation 39 minLecture4.5
-
Concentration cell, Battery, Corrosion 52 minLecture4.6
-
Electrolysis 20 minLecture4.7
-
Faraday Law 45 minLecture4.8
-
Resistance & Conductance 40 minLecture4.9
-
Molar & Eq. Conductance, Kohlraush’s Law 29 minLecture4.10
-
Problems on Resistance & Conductance 23 minLecture4.11
-
Chapter Notes – ElectrochemistryLecture4.12
-
NCERT Solutions – ElectrochemistryLecture4.13
-
-
5. Surface Chemistry
11-
Introduction & Surface tension & surface energy 33 minLecture5.1
-
Adsorption 47 minLecture5.2
-
Factors affecting Adsorption 39 minLecture5.3
-
Catalysis 34 minLecture5.4
-
Type of Catalysis & Enzyme Catalysis 41 minLecture5.5
-
Colloidal Solution 57 minLecture5.6
-
Type of Colloidal Solution 43 minLecture5.7
-
Properties of Colloidal Solution 50 minLecture5.8
-
Protective Colloids 58 minLecture5.9
-
Chapter Notes – Surface ChemistryLecture5.10
-
NCERT Solutions – Surface ChemistryLecture5.11
-
-
6. Alcohol & Ether
8-
Preparation 35 minLecture6.1
-
Physical Properties & Oxidation Of Alcohol 29 minLecture6.2
-
Hydrates, Acetal, Ketal 38 minLecture6.3
-
Tests Of Alcohol 47 minLecture6.4
-
Ether Preparation & Its Properties 33 minLecture6.5
-
Thiol & Thioether 16 minLecture6.6
-
Chapter Notes – Alcohol & EtherLecture6.7
-
NCERT Solutions – Alcohol & EtherLecture6.8
-
-
7. Aldehyde & Ketone
10-
Preparation 33 minLecture7.1
-
Physical Properties, Beckmann Rearrangement, Witting Reaction 46 minLecture7.2
-
Schmidt Reaction, Bayer Villegar Oxidation 22 minLecture7.3
-
Aldol Condensation Reaction 40 minLecture7.4
-
Cannizzaro Reaction 32 minLecture7.5
-
Acyloin, Benzoin, Clasien, Perkin Condensation 28 minLecture7.6
-
Reformasky Reaction, Tischenko Reaction 20 minLecture7.7
-
Tests-8 40 minLecture7.8
-
Chapter Notes – Aldehyde & KetoneLecture7.9
-
NCERT Solutions – Aldehyde & KetoneLecture7.10
-
-
8. Acid & derivatives
4-
Preparation 31 minLecture8.1
-
Chemical Reactions Of Acids 31 minLecture8.2
-
Arndt Eistert, Curtius, Hvz, Hoffmann Reaction 19 minLecture8.3
-
Acid Derivatives 38 minLecture8.4
-
-
9. Nitrogen containing compounds
4-
Alkyl Nitrites, Nitro Alkane 27 minLecture9.1
-
Alkane Nitrile & Isonitrile 20 minLecture9.2
-
Amine Preparation 24 minLecture9.3
-
Properties Of Amines 13 minLecture9.4
-
-
10. Aromatic Compounds
7-
Benzene 41 minLecture10.1
-
Aromatic Hydrocarbon 29 minLecture10.2
-
Aryl Halides 18 minLecture10.3
-
Phenol 40 minLecture10.4
-
Aromatic Aldehyde 39 minLecture10.5
-
Aniline 32 minLecture10.6
-
Phenyl Diazonium Salts 37 minLecture10.7
-
-
11. Biomolecules
14-
Introduction & Types Of Carbohydrates 47 minLecture11.1
-
D-glucose & D-fructose 50 minLecture11.2
-
Reactions Of D-glucose & D-fructose 32 minLecture11.3
-
Reactions Of D-glucose & D-fructose 23 minLecture11.4
-
Sucrose, Maltose, Lactose 31 minLecture11.5
-
Starch, Cellulose, Glycogen 27 minLecture11.6
-
Reducing Sugar, Mutarotation, Osazone Formation 40 minLecture11.7
-
Problems On Carbohydrates 41 minLecture11.8
-
Amino Acids 48 minLecture11.9
-
Peptides 47 minLecture11.10
-
Proteins 18 minLecture11.11
-
Enzyme & Vitamins 30 minLecture11.12
-
Nucleic Acid 36 minLecture11.13
-
Chapter Notes – BiomoleculesLecture11.14
-
-
12. Polymer Chemistry
6-
Polymerisation Addition Reaction 32 minLecture12.1
-
Coordination Addition, Condensation Reaction 24 minLecture12.2
-
Division Of Polymer 41 minLecture12.3
-
Examples Of Polymer 31 minLecture12.4
-
Examples Of Polymer 31 minLecture12.5
-
Chapter Notes – Polymer ChemistryLecture12.6
-
-
13. Practical Organic Chemistry
4-
Poc Qualitative Analysis 23 minLecture13.1
-
Poc Qualitative Analysis 20 minLecture13.2
-
Poc Quantitative Analysis 29 minLecture13.3
-
Poc Quantitative Analysis 20 minLecture13.4
-
-
14. P block elements II
13-
VA – Elemental Properties of N family 51 minLecture14.1
-
VA – Compounds of N family 43 minLecture14.2
-
VA – N & Its compounds 45 minLecture14.3
-
VA – Oxides & Oxyacids of Nitrogen 55 minLecture14.4
-
VA – P & its compounds 31 minLecture14.5
-
VA – Oxides & Oxyacids of P 31 minLecture14.6
-
VIA 1 – Elemental Properties of O-Family 36 minLecture14.7
-
VIA 2 – compounds of VIA elements 41 minLecture14.8
-
VIA 3 – Oxygen & Ozone 47 minLecture14.9
-
VIA 4 – Sulphur & oxides of Sulphur 37 minLecture14.10
-
VIA 5 – Sulphuric Acid 25 minLecture14.11
-
Chapter Notes – P block elementsLecture14.12
-
NCERT Solutions – P block elementsLecture14.13
-
-
15. P block elements III
5-
VIIA 1 – elemental properties of Halogen 40 minLecture15.1
-
VIIA 2 – Compounds of Halogen 49 minLecture15.2
-
VIIA 3 – Chlorine & its Compounds 41 minLecture15.3
-
VIIIA 1 – Properties of Noble Gas 34 minLecture15.4
-
VIIIA 2 – Compounds of Noble Gas 34 minLecture15.5
-
-
16. D block metals
8-
D block – Elemental Properties 55 minLecture16.1
-
Elemental Properties 01 hourLecture16.2
-
Elemental Properties 53 minLecture16.3
-
KMnO4 & K2Cr2O7 47 minLecture16.4
-
Problems 40 minLecture16.5
-
Problems 20 minLecture16.6
-
Chapter Notes – The d-and f-Block ElementsLecture16.7
-
NCERT Solutions – The d-and f-Block ElementsLecture16.8
-
-
17. F block metals
3-
Lanthanoids 52 minLecture17.1
-
Actinoids 48 minLecture17.2
-
Problems 42 minLecture17.3
-
-
18. Co-ordination compounds
17-
Introduction of Complex Compound, Ligands 42 minLecture18.1
-
Classification of Ligands, Denticity 35 minLecture18.2
-
Nomenclature of Complex Compounds 46 minLecture18.3
-
Nomenclature of Complex Compounds 2 40 minLecture18.4
-
Bonding in Complex Compound, Primary & Secondary Valency 44 minLecture18.5
-
Concept of EAN 29 minLecture18.6
-
VBT in Complex Compounds 58 minLecture18.7
-
Examples on VBT in complex compounds 31 minLecture18.8
-
CFT in Complex Compounds 43 minLecture18.9
-
CFT for Octahedral & Tetrahedral Complex 35 minLecture18.10
-
Colour & Stability of Complex Compounds 28 minLecture18.11
-
Structural Isomerism in Complex Compounds 49 minLecture18.12
-
Geometrical Isomerism in Complex Compounds 43 minLecture18.13
-
Optical Isomerism in Complex Compounds, use of Complex 01 hourLecture18.14
-
Organometallic Compounds 29 minLecture18.15
-
Chapter Notes – Co-ordination compoundsLecture18.16
-
NCERT Solutions – Co-ordination compoundsLecture18.17
-
-
19. Environmental Chemistry
4-
Introduction & Air Pollution 35 minLecture19.1
-
Air Pollution 20 minLecture19.2
-
Water Pollution 23 minLecture19.3
-
Soil Pollution, Prevention of Pollution 16 minLecture19.4
-
Chapter Notes – Electrochemistry
Electrochemistry is that branch of chemistry that deals with the study of the production of electricity from the energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations.
Importance of Electrochemistry
1. Production of metals like Na, Mg. Ca and Al.
2. Electroplating.
3. Purification of metals.
4. Batteries and cells used in various instruments.
Conductors
Substances that allow electric current to pass through them are known as conductors.
Metallic Conductors or Electronic Conductors
Substances which allow the electric current to pass through them by the movement of lectrons are called metallic conductors, e.g.. metals.
Electrolytic Conductors or Electrolytes
Substances which allow the passage of electricity through their fused state or aqueous solution and undergo chemical decomposition are called electrolytic conductors, e.g., aqueous solution of acids. bases and salts.
Electrolytes are of two types:
1. Strong electrolytes
The electrolytes that completely dissociate or ionise into ions are called strong electrolytes. e.g., HCl, NaOH, K2SO4
2. Weak electrolytes
The electrolytes that dissociate partially (ex < 1) are called weak electrolytes, e.g., CH3COOH, H2CO3, NH4OHH2S, etc.
Electrochemical Cell and Electrolytic
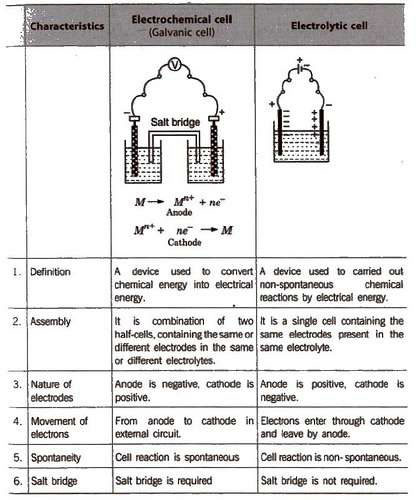
A cell of almost constant emf is called standard cell. The most common is Weston standard cell. Galvanic cell is also called voltaic cell.
General Representation of an Electrochemical Cell
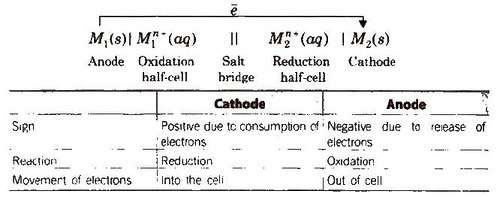
Other features of the electrochemical cell are
1. There is no evolution of heat.
2. The solution remains neutral on both sides.
3. The reaction and now of electrons stops after sometime.
Daniell Cell
An electrochemical cell of zinc and copper metals is known as Daniell cell. It is represented as
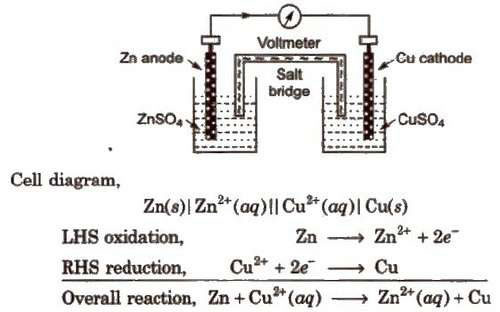
By convention cathode is represented on the RHS and anode on the LHS.
Function of salt bridge
1. It completes the circuit and allows the flow of current.
2. It maintains the electrical neutrality on both sides. Salt-bridge generally contains solution of strong electrolyte such as KNO3, KCL etc. KCI is preferred because the transport numbers of K+ and Cl-are almost same.
Transport number or Transference number
The current flowing through an electrolytic solution is carried by the ions. The fraction of the current carried by an ion is called its transport number or transference number. Thus.
Transport number of cation. nc = (current carried by cation/total current)
Transport number of cation. na = (current carried by anion/total current)
Evidently nc + na = 1
Electrode Potential
When an electrode is in contact with the solution of its ions in a half-cell, it has a tendency to lose or gain electrons which is known as electrode potential. It is expressed in volts. It is an intensive property, i.e., independent of the amount of species in the reaction.
Oxidation potential
The tendency to lose electrons in the above case is known as oxidation potential. Oxidation potential of a half-cell is inversely proportional to the concentration of ions in the solution.
Reduction potential
The tendency to gain electrons in the above case is known as reduction potential. According to IUPAC convention, the reduction potential alone be called as the electrode potential unless it is specifically mentioned.
E°red = – E°oxidalion
It is not possible to determine the absolute value of electrode potential. For this a reference electrode [NHE or SHE] is required. The electrode potential is only the difference of otentials between two electrodes that we can measure by combining them to give a complete cell.
Standard electrode potential
The potential difference developed between metal electrode and solution of ions of unit molarity (1M) at 1 atm pressure and 25°C (298 K) is called standard electrode potential.
It is denoted by E°.
Reference Electrode
The electrode of known potential is called reference electrode. It may be primary reference electrode like hydrogen electrode or secondary reference electrode like calomel electrode.
Standard hydrogen electrode (SHE) Standard hydrogen electrode (SHE). also known as normal hydrogen electrode (NHE), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The wire is sealed into a glass tube. placed in beaker containing 1 M HCl. The hydrogen gas at 1 atm pressure is bubbled through the solution at 298K. Half-cell is pt H2 (1 atm) H+ (1 M)
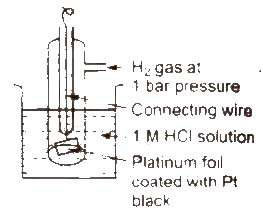
In SHE. at the surface of plantinum, either of (he following reaction can take place
2H+(ag) + 2e- → H2G Reduction
H2(g) → 2H+(ag) + 2e- Oxidation
The electrode potential of SHE has been fixed as zero at all temperatures.
Its main drawbacks are
1. It is difficult to maintain 1 atm pressure of H2 gas.
2. It is difficult to maintain H+ ion concentration 1 M.
3. The platinum electrode is easily poisoned by traces of impurities.
Hence, calomel electrodes are conveniently used as reference electrodes, It consists of mercury in contact with Hg2 Cl2 (calomel) paste in a solution of KCl.
Electromotive Force (emf) of a Cell
It is the difference between the electrode potentials of two half-cells and cause flow of current from electrode at higher potential to electrode at lower potential. It is also the measure of free energy change. Standard emf of a cell,
![]()
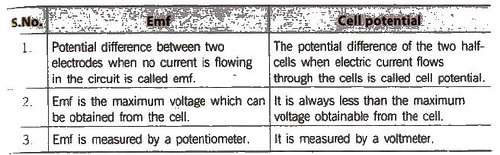
Electrochemical Series
It is the arrangement of electrodes in the increasing order of their standard reduction potentials.
Standard Electrode Potential at 298 K
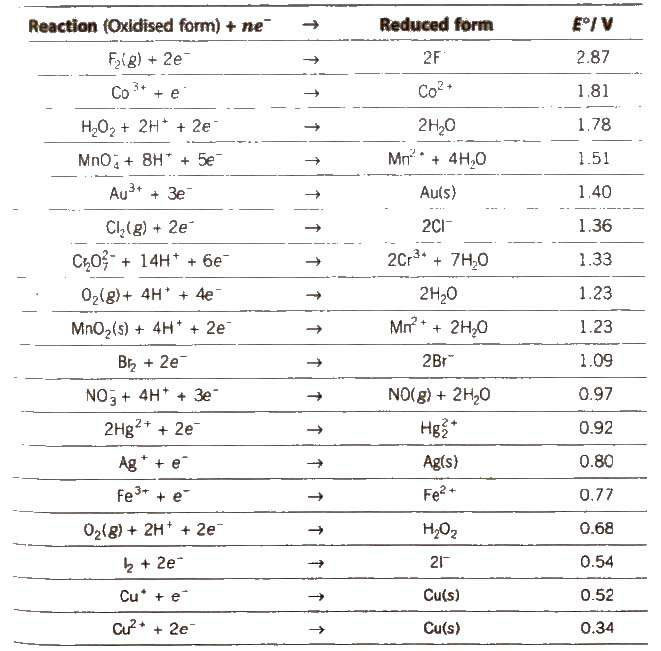
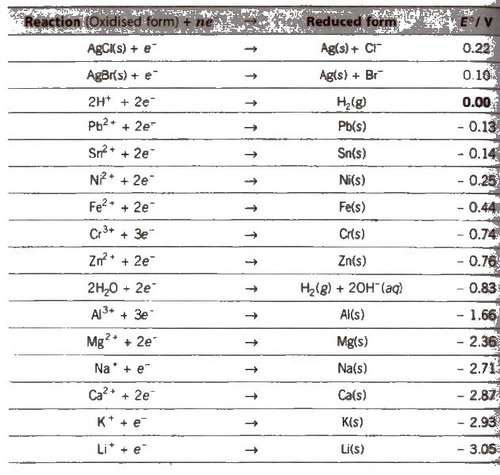
Appications of Electrochemical Series (ECS)
1. The lower the value of E°, the greater the tendency to form cation.
M → Mn+ + ne–
Metals placed below hydrogen in ECS replace hydrogen from di1 acids but metals placed above hydrogen cannot replace hydrogen from dil acids.
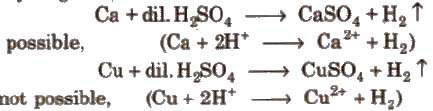
3. Oxides of metals placed below hydrogen are not reduced by H2 but oxides of iron and metals placed above iron are reduced by H2·
•SnO, PbO, CuO are reduced by H2
•CaO, K2O are not reduced by H2·
4. Reducing character increases down the series.
5. Reactivity increases down the series.
6. Determination of emf; emf is the difference of reduction potentials of two half-cells.
•Eemf = ERHS – ELHS
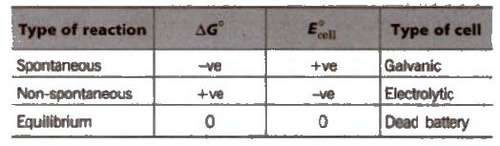
If the value of emf is positive. then reaction take place spontaneously, otherwise not.
7. Greater the reduction potential of a substance, oxidising power. (e.g.. F2 > Cl2 > Br2 > I2)
8. A negative value of standard reduction potential shows that it is the site of oxidation.
9. Oxides of metals having E°red ≥ 0.79 will be decomposed by heating to form O2 and metal.
HgO (s) → Hg(l)(1/2)O2(g)
(E°Hg 2+ /Hg = 0.79V)
Nernst Equation
The relationship between the concentration of ions and electrode potential is given by Nernst equation.
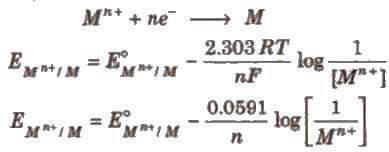
For a electrochemical cell,
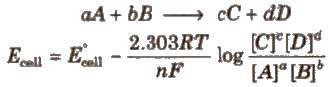
Concentration of pure solids and liquids is taken as unity.
Nernst equation and Kc
At equilibrium
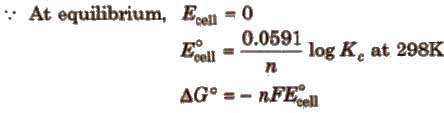
Here, ΔG° is the standard Gibbs free energy change.
Relationship between free energy change and equilibrium constant
ΔG° = – 2.303RT log Kc
Concentration Cells
(i) Electrode concentration cells Two hydrogen electrodes or different pressures are dipped In the same solution of electrolyte, e.g..
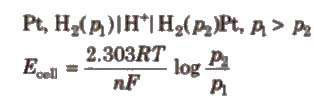
(ii) Electrolyte concentration cells Electrodes are the same but electrolyte solutions have different concentrations, e.g..
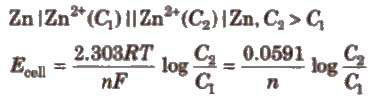
Conductance (G)
It is the ease of flow of electric current through the conductor. It is reciprocal of resistance (R).
G = (1/R), units ohm-1 mhos or Ω-1
Specific Conductivity (K)
It is the reciprocal of specific resistance.
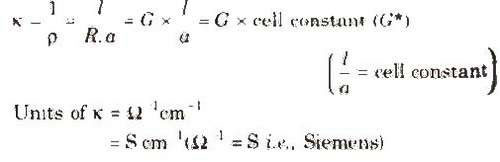
Unit of cell constant is cm-1 or m-1.
Specific conductivity decreases on dilution. This is because concentration of ions per cc decreases upon dilution.
Molar Conductivity (Λm)
The conductivity of all the ions produced when 1 mole of an electrolyte is dissolved in V mL of solution is known as molar conductivity.
It is related to specific conductance as
Λm = (k x 1000/M)
where. M = molarity.
It units are Ω-1 cm2 mol-1 or S cm2 mol-1.
Equivalent conductivity (Λm)
The conducting power of all the ions produced when 1 g-equivalent of an electrolyte is dissolved in V mL of solution, is called equivalent conductivity. It is related to specific conductance as
Λm = (k x 1000/N)
where. N = normality.
Its units are ohm-1 cm2 (equiv-1) or mho cm2 (equiv-1) or S cm2 (g-equiv-1).
Debye-Huckel Onsagar equation
It gives a relation between molar conductivity, Λm at a particular concentration and molar conductivity Λm at infinite dilution.
Λm = Λ0 m – √C
where, b is a constant. It depends upon the nature of solvent and temperature.
Factors Affecting Conductivity
(i) Nature of electrolyte
The strong electrolytes like KNO3 KCl. NaOH. etc. are completely ionised in aqueous solution and have high values of conductivity (molar as well as equivalent).
The weak electrolytes are ionised to a lesser extent in aqueous solution and have lower values of conductivity (molar as well as equivalent) .
ii) Concentration of the solution
The concentrated solutions of strong electrolytes have SIgnificant interionic attractions. which reduce the speed of ions and lower the value of Λm. and Λeq.
The dilution decreases such attractions and increase the value of Λm and Λeq.
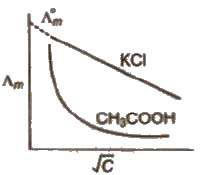
The limiting value, Λ0 m or Λ∞ m. (the molar conductivity at zero concentration (or at infinite dilution) can be obtained extrapolating the graph.
In case of weak electrolytes, the degree of ionisation increases dilution which increases the value of Λ m and Λeq. The liminting value Λ0 m cannot be obtained by extrapolating the graph. ~ limiting value, Λ0 m, for weak electrolytes is obtained by Kohlrausch law.
(iii) Temperature The increase of temperature decreases inter-ionic attractions and increases kinetic energy of ions and their speed. Thus, Λm and Λeq increase with temperature.
Kohlrausch’ s Law
At infinite dilution, the molar conductivity of an electrolyte is the sum of the ionic conductivities of the cations and anions, e.g., for AxBy.
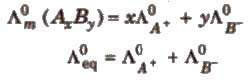
Applications
(i) Determination of equivalent/molar conductivities of weak electrolytes at infinite dilution,e.g.,
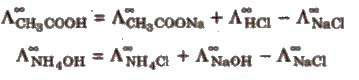
(ii) Determination of degree of dissociation (α) of an electrolyte at a given dilution.

The dissociation constant (K) of the weak electrolyte at concentration C of the solution can be calculated by using the formula
kc = (Cα2/1 – α)
where, α is the degree of dissociation of the electrolyte.
(iii) Salts like BaSO4 .., PbSO4‘ AgCl, AgBr and AgI which do not dissolve to a large extent in water are called sparingly soluble salts.
The solubility of a sparingly soluble salt can be calculated as
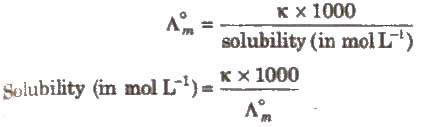
Electrolysis
It is the process of decomposition of an electrolyte when electric current is passed through either its aqueous solution or molten state,
1. In electrolytic cell both oxidation and reduction takes place in the same cell.
2. Anode is positively charged and cathode is negatively charged, In electrolytic cell.
3. During electrolysis of molten electrolyte, cations are liberated at cathode. while anions at the anode.
4. When two or more ions compete at the electrodes. the ion with higher reduction potential gets liberated at the cathode while the ion with lower reduction potential at the anode.
For metals to be deposited on the cathode during electrolysis, the voltage required is almost the same as the standard electrode potential. However for liberation of gases, some extra voltage is required than the theoretical value of the standard electrode potential. The extra voltage thus required is called over voltage or bubble voltage.
How to Predict the Products of Electrolysis?
When an aqueous solution of an electrolyte is electrolysed, if the cation has higher reduction potential than water (-0.83 V), cation is liberated at the cathode (e.g.. in the electrolysis of copper and silver salts) otherwise H2 gas is liberated due to reduction of water (e.g., in the electrolysis of K, Na, Ca salts, etc.)
Similarly if anion has higher oxidation potential than water (- 1.23 V), anion is liberated (e.g., Br-), otherwise O2 gas is liberated due to oxidation of water (e.g., in caseof F-, aqueous solution of Na2SO4 as oxidation potential of SO2- 4 is – 0.2 V).
Discharge potential is defined as the minimum potential that must be applied acrossthe electrodes to bring about the electrolysis and subsequent discharge of the ion on the electrode.
Faraday’s Laws of Electrolysis
1. First law
The amount of the substance deposited or liberated at cathode directly proportional to the quantity of electricity passed through electrolyte.
W ∝ I x t = I x t x Z = Q x Z
•I current in amp, t = time in sec,
•Q = quantity of charge (coulomb)
•Z is a constant known as electrochemical equivalent.
When I = 1 amp, t = 1 sec then Q = 1 coulomb, then w = Z.
Thus, electrochemical equivalent I” the amount of the substance deposited or liberated by passing 1A current for 1 sec (i.e.. 1 coulomb, I x t = Q)
2. Second law
When the same quantity of electricity is passed through different electrolytes. the amounts of the substance deposited or liberated at the electrodes arc directly proportional to their equivalent weights, Thus,
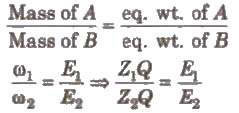
Hence, electrochemical equivalent ∝ equivalent weight.
Batteries
These are source of electrical energy which may have one or more cells connected in series. For a good quality battery it should be reasonably light. compact and its voltage should not vary appreciably during its use.
Primary Batteries
In the primary batteries. the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again.
(i) Dry cell or Leclanehe cell
Anode-Zinc container
Cathode-Graphite rod surrounded by MnO2 powder
Electrolyte-Paste of NH4Cl + ZnCl2
Cathode reaction,
2MnO2(s) + 2 NH+4(aq) + 2e- → Mn2O3(s) + 2NH3(g) + H2O(l)
Anode reaction,
Zn(s) → Zn2+(aq) + 2e–
Cell potential 1.25 V to 1.5 V
(ii) Mercury cell
Anode-Zn-Hg amalgam
Cathode-Paste of (HgO + C)
Electrolyte-Moist paste of KOH-ZnO
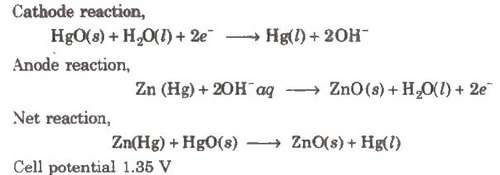
Secondary Batteries
These cells can be recharged and can be used again and again, e.g.,
(i) Lead Storage battery
Anode-Spongy lead
Cathode-Grid of lead packed with PbO2
Electrolyte-38% H2SO4 by mass
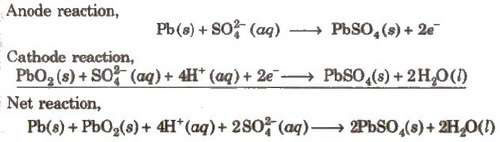
When recharged the cell reactions are reversed.
(ii) Nickel-cadmium storage cell
Anode-Cadmium
Cathode-Metal grid containing NiO2 Electrolyte-KOH solution
Anode reaction,
Cd(s) + 2OH–(aq) → Cd(OH)2(s) + 2e–
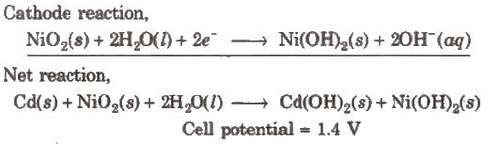
Fuel Cells
Galvanic cells which use energy of combustion of fuels like H2, CH4, CH3OH, etc., as the source to produce electrical energy are called fuel cells. The fuel cells are pollution free and have high efficiency.
Hydrogen-Oxygen Fuel Cell
Electrodes-Made of porous graphite impregnated with catalyst (Pt, Ag or a metal oxide).
Electrolyte-Aqueous solution of KOH or NaOH
Oxygen and hydrogen are continuously fed into the cell.
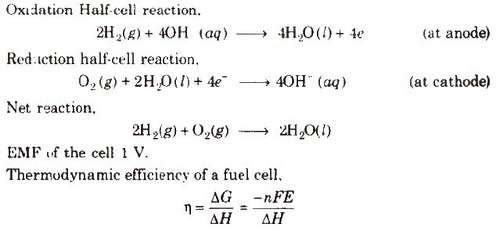
Corrosion
Slow formation of undesirable compounds such as oxides, sulphides or carbonates at the surface of metals by reaction with moisture and other atmospheric gases is known as orrosion.
Factors Affecting Corrosion
1. Reactivity of metals
2. Presence of moisture and atmospheric gases like CO2, SO2, etc.
3. Presence of impurities
4. Strains in the metal
5. Presence of electrolyte
Rusting of Iron-Electrochemical Theory
An electrochemical cell, also known as corrosion cell, is developed at the surface of iron.
Anode- Pure iron
Cathode-Impure surface
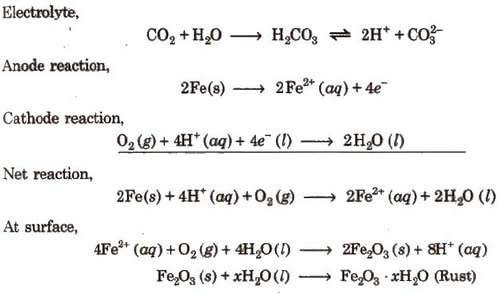
Rusting of iron can be prevented by the following methods :
1. Barrier protection through coating of paints or electroplating.
2. Through galvanisation or coating of surface with tin metal.
3. By the use of antirust solutions (bis phenol).
4. By cathodic protection in which a metal is protected from corrosion by connecting it to another metal that is more easily oxidised.
