-
1. Stoichiometry 1
13-
Introduction & POAC 30 minLecture1.1
-
Mole Stoichiometric relationship 29 minLecture1.2
-
Successive reaction & Limiting reagent 25 minLecture1.3
-
Gas Stoichiometry 29 minLecture1.4
-
Important Types of Reactions 25 minLecture1.5
-
Avogadro’s No.1 30 minLecture1.6
-
Mole & Number 28 minLecture1.7
-
Atomic, Molecular Wt 26 minLecture1.8
-
Ionic wt, Avg. At. Wt. 15 minLecture1.9
-
Molar wt. 27 minLecture1.10
-
Molar Volume & Gas Analysis 30 minLecture1.11
-
Gas Analysis 17 minLecture1.12
-
Empirical Formula Determination 26 minLecture1.13
-
-
2. Stoichiometry 2
18-
Acid Base definition 23 minLecture2.1
-
Acidity & Basicity 32 minLecture2.2
-
Acidic Strength 30 minLecture2.3
-
Acidic Strength 23 minLecture2.4
-
Conjugate Acid-Base pair, Basic Strength 48 minLecture2.5
-
Oxidation & Reduction 50 minLecture2.6
-
Calculation of Oxidation Number 46 minLecture2.7
-
O.A. & R.A., Balancing by Oxidation Number Method 01 hourLecture2.8
-
Balancing by Ion Electron Method. 35 minLecture2.9
-
Eq. Wt. 1 – n factor & Eq. Wt. Concept 47 minLecture2.10
-
Eq. Wt. 2 – Eq. Concept 35 minLecture2.11
-
Volumetric Analysis 43 minLecture2.12
-
Volumetric analysis 44 minLecture2.13
-
Titration – Acid Base Titration 49 minLecture2.14
-
Titration – Acid Base Titration, Indicator 56 minLecture2.15
-
Titration – Redox Titration-8 58 minLecture2.16
-
Titration – Redox Titration, volume Strength of H2O2 50 minLecture2.17
-
Titration – Redox Titration, Iodometry, Oleum, Bleaching Powder 49 minLecture2.18
-
-
3. Thermodynamics & Thermochemistry
19-
Zeroth Law 55 minLecture3.1
-
1st law – System, Properties, State 40 minLecture3.2
-
1st Law, Process, Internal energy, Work 43 minLecture3.3
-
Work done in Irreversible process, Isobaric Process 49 minLecture3.4
-
Isochoric Process & problems TD 42 minLecture3.5
-
Isothermal irreversible Process, Problems on TD 46 minLecture3.6
-
Adiabatic Process 49 minLecture3.7
-
Problems on TD 44 minLecture3.8
-
Thermochemistry & Enthalpy 38 minLecture3.9
-
Hess’s Law, Kirchhoff’s Law 43 minLecture3.10
-
Enthalpy of Formation, combustion 39 minLecture3.11
-
Enthalpy of Hydrogenation, Hydration, dissolution, lattice energy 40 minLecture3.12
-
Enthapy of Neutralisation, atomisation, Bond Energy 47 minLecture3.13
-
Resonance energy & problems 45 minLecture3.14
-
2nd Law, Entropy-positional 40 minLecture3.15
-
TD Entropy, 3rd Law, Entropy change in a reaction 45 minLecture3.16
-
Gibb’s free energy 43 minLecture3.17
-
Efficiency, engine, pump & Carnot engine 39 minLecture3.18
-
Chapter Notes – Thermodynamics & ThermochemistryLecture3.19
-
-
4. Atomic Structure
22-
Introduction, Cathode rays & Anode rays 41 minLecture4.1
-
J.J. Thomson Model, Millikan Oil Drop Experiment 38 minLecture4.2
-
Rutherford Experiment 51 minLecture4.3
-
Quantum Mechanics, BlackBody Radiation Experiment 41 minLecture4.4
-
Wave 44 minLecture4.5
-
Photoelectric Effect 46 minLecture4.6
-
Problems on Photoelectric Effect 35 minLecture4.7
-
Atomic Structure 44 minLecture4.8
-
Bohr Theory 47 minLecture4.9
-
H – Spectrum 49 minLecture4.10
-
Problems on Bohr’s Theory 40 minLecture4.11
-
Adv. Problems on Bohr Theory & Sommerfeld model 51 minLecture4.12
-
Quantum Mechanical Model for Atomic Structure 47 minLecture4.13
-
Schrodinger wave equation 54 minLecture4.14
-
No. of Orbitals & Quantum no 45 minLecture4.15
-
Orbital Curve, RPD curve, Definition of Node 46 minLecture4.16
-
Calculation of Node, Orbital Picture 43 minLecture4.17
-
Radial Probability curve, MPD, Avg. distance, Screening effect, Zeff 38 minLecture4.18
-
Multielectron system, Electronic configuration 56 minLecture4.19
-
Stability of Elec. Configuration 36 minLecture4.20
-
Chapter Notes – Atomic StructureLecture4.21
-
NCERT Solutions – Atomic StructureLecture4.22
-
-
5. Chemical equilibrium
9-
Introduction, Eqb constant & Eqb Position 51 minLecture5.1
-
Types of Eqb Constant, Heterogeneous Eqb, Reaction Quotient 45 minLecture5.2
-
Range of Eqb Constant 43 minLecture5.3
-
Problems on Chemical Eqb 41 minLecture5.4
-
Problems on Chemical Eqb 42 minLecture5.5
-
Le-chatelier Principle 42 minLecture5.6
-
Le-Chatelier Principle 35 minLecture5.7
-
Eqb & 2nd Law of TD 26 minLecture5.8
-
NCERT Solutions – equilibriumLecture5.9
-
-
6. Ionic Equilibrium
17-
Electrolyte, Dissociation of H2O, Nature of Solution 46 minLecture6.1
-
PH scale, Log & Antilog 40 minLecture6.2
-
PH of Strong Acid, Base Solution 51 minLecture6.3
-
PH of Weak Acid, Base solution 41 minLecture6.4
-
PH of mixture of Acids, Bases 46 minLecture6.5
-
PH of Polybasic acids 40 minLecture6.6
-
PH of Salt Solution 1 43 minLecture6.7
-
PH of salt solution 2 52 minLecture6.8
-
Common ion effect, Buffer solution 49 minLecture6.9
-
Buffer Capacity 45 minLecture6.10
-
Titration & PH Curve 1 40 minLecture6.11
-
Titration & PH curve 2 46 minLecture6.12
-
Acid Base indicator 35 minLecture6.13
-
Solubility Equilibrium 47 minLecture6.14
-
Precipitation of Solid, Qualitative analysis of cation 44 minLecture6.15
-
Complex ion equilibrium 23 minLecture6.16
-
Chapter Notes – EquilibriumLecture6.17
-
-
7. Introduction & Development of Org. Chemistry
3-
Introduction & Development Of Organic Chemistry 44 minLecture7.1
-
Introduction & Syllabus 36 minLecture7.2
-
NCERT Solutions – Org. ChemistryLecture7.3
-
-
8. Nomenclature of Org. Compounds
16-
Alkane 59 minLecture8.1
-
Alkane 31 minLecture8.2
-
Alkyl Group & Types Of Hydrogen 01 hourLecture8.3
-
Alkene 54 minLecture8.4
-
Alkenyl 32 minLecture8.5
-
Alkyne & Alkenyl 47 minLecture8.6
-
Cycloalkane 43 minLecture8.7
-
Cycloalkene 35 minLecture8.8
-
Bicycloalkane & Spirane 35 minLecture8.9
-
Acid & Aldehyde 45 minLecture8.10
-
Ester & Acid Halides 28 minLecture8.11
-
Amide & Nitrile 28 minLecture8.12
-
Alcohol & Sulphonic Acid 37 minLecture8.13
-
Isonitrile, Amine, Nitroalkane, Halo Compounds 39 minLecture8.14
-
Ketone, Anhydride & Ether 34 minLecture8.15
-
Polyfunctional Group Compounds 41 minLecture8.16
-
-
9. GOC 1- Hybridisation, Resonance, Aromaticity
16-
Concept Of Hybridisation 42 minLecture9.1
-
Sp3, Sp2 Hybridisation 44 minLecture9.2
-
Sp Hybridisation, Relative Study Of Sp3, Sp2, Sp Orbitals 46 minLecture9.3
-
Effect Of Hybridisation On Bond Length, Planar Nature 59 minLecture9.4
-
Concept Of Resonance 39 minLecture9.5
-
Doing Resonance 18 minLecture9.6
-
Resonance Hybrid, Cannonical St. , Resonance Energy 44 minLecture9.7
-
Condition Of Resonance 40 minLecture9.8
-
Writing Cannonical St. 39 minLecture9.9
-
Relative Stability Of Cannonical St. 37 minLecture9.10
-
Resonance Energy 45 minLecture9.11
-
Effect Of Resonance On Bond Length, Enthalpy Of Hydrogenation 43 minLecture9.12
-
Introduction To Aromaticity 43 minLecture9.13
-
Introduction To Aromaticity 39 minLecture9.14
-
Unsaturation Factor 31 minLecture9.15
-
Chapter Notes – GOC General Organic chemistryLecture9.16
-
-
10. GOC 2 - Substituent effect
5-
Substituent Effect, Hyperconjugation 48 minLecture10.1
-
Substituent Effect, Hyperconjugation 43 minLecture10.2
-
Substituent Effect, Mesomeric Effect 47 minLecture10.3
-
Substituent Effect, Inductive Effect 46 minLecture10.4
-
Substituent Effect, Electromeric Effect, Staric Effect, Relative M & I Effect 41 minLecture10.5
-
-
11. GOC 2 - Reactive Intermediate
6-
Reactive Intermediate, Carbocation 45 minLecture11.1
-
Reactive Intermediate, Carbocation, Carbonium Ion Rearrangement 42 minLecture11.2
-
Reactive Intermediate, Carbonium Ion Rearrangement 41 minLecture11.3
-
Reactive Intermediate, Carbanion 36 minLecture11.4
-
Reactive Intermediate, Free Radical 47 minLecture11.5
-
Reactive Intermediate, Carbene & Nitrene 42 minLecture11.6
-
-
12. GOC 2 - Acid, base, Electrophile, Nucleophile
3-
Acid Base, Electrophile Nucleophile 50 minLecture12.1
-
Acid Base, Electrophile Nucleophile 47 minLecture12.2
-
Hard Acid Base, Electrophilic Nucleophilic Strength 40 minLecture12.3
-
-
13. Isomerism
20-
Structural Isomers 39 minLecture13.1
-
Tautomerism 37 minLecture13.2
-
Stability Of Tautomers 43 minLecture13.3
-
Factors Affecting Stability, Catalysis In Tautomerism 39 minLecture13.4
-
Geometrical Isomerism 41 minLecture13.5
-
E-z Nomenclature, Properties Of G.i. 43 minLecture13.6
-
No. Of G.i., Interconversion Of G.i. 48 minLecture13.7
-
Optical Isomerism & Its Conditions 50 minLecture13.8
-
Different Types Of Projections, R-s Configuration 57 minLecture13.9
-
Relationship Between Optical Isomers 45 minLecture13.10
-
Dissymmetry In A Molecule 44 minLecture13.11
-
Enantiomers, Mesomers, Diastereomers 39 minLecture13.12
-
Special Case Of Optical Isomerism 47 minLecture13.13
-
No. Of Optical Isomers, Stereoisomers 45 minLecture13.14
-
D,l Configuration, Retention & Inversion 36 minLecture13.15
-
Measurement Of Optical Activity 45 minLecture13.16
-
No. Of Isomers 35 minLecture13.17
-
Resolution Of Optical Isomers, Syn, Anti Addition, Elimination. 28 minLecture13.18
-
Conformational Isomers 51 minLecture13.19
-
Conformers Of Propane, Butane, Cyclohexane & Problems 44 minLecture13.20
-
-
14. Reaction Mechanism
21-
Introduction, Types Of Organic Reactions 35 minLecture14.1
-
Nucleophilic Substitution Reaction 40 minLecture14.2
-
Sn1 & Sn2 Reaction, Sni Pathway 53 minLecture14.3
-
Reactivity In Sn1 & Sn2 Path 42 minLecture14.4
-
Reactivity In Sn1 & Sn2 Path 36 minLecture14.5
-
Reactivity In Sn1 & Sn2 Path 30 minLecture14.6
-
Reactivity In Sn1 & Sn2 Path 41 minLecture14.7
-
Elimination Reaction 53 minLecture14.8
-
E1 & E2 Reaction, Isotopic Effect 46 minLecture14.9
-
Orientation In Elimination Reaction 45 minLecture14.10
-
Problems On Elimination Reaction 48 minLecture14.11
-
Elimination Vs Substitution 34 minLecture14.12
-
Addition Reaction 51 minLecture14.13
-
Problems On Addition Reaction 46 minLecture14.14
-
Electrophilic Aromatic Substitution Reaction 49 minLecture14.15
-
Orientation In Electrophilic Aromatic Substitution 53 minLecture14.16
-
Reactivity In Electrophilic Aromatic Substitution Reaction 30 minLecture14.17
-
Examples Of Electrophilic Aromatic Substitution Reaction 37 minLecture14.18
-
Examples Of Electrophilic Aromatic Substitution Reaction 37 minLecture14.19
-
Nucleophilic Aromatic Substitution 44 minLecture14.20
-
Benzyne Pathway 27 minLecture14.21
-
-
15. Alkane
7-
Alkane Preparation 49 minLecture15.1
-
Alkane Preparation & Selective Hydrogenation 31 minLecture15.2
-
Alkane Preparation 40 minLecture15.3
-
Alkane Preparation 38 minLecture15.4
-
Alkane Preparation 32 minLecture15.5
-
Alkane Properties 55 minLecture15.6
-
Alkane Properties & Problems 39 minLecture15.7
-
-
16. Alkene
7-
Alkene Preparation 45 minLecture16.1
-
Alkene Preparation 36 minLecture16.2
-
Alkene Properties 53 minLecture16.3
-
Alkene Properties 40 minLecture16.4
-
Alkene Properties 42 minLecture16.5
-
Alkene Properties & Ozonolysis 41 minLecture16.6
-
Alkene Properties, Oxidation, Substitution 38 minLecture16.7
-
-
17. Alkyl Halides
4-
Preparation 38 minLecture17.1
-
Properties 49 minLecture17.2
-
Haloform Reaction 28 minLecture17.3
-
Grignard Reagent 29 minLecture17.4
-
-
18. Chemical Bonding
32-
Introduction, definition, Concept & Type of Bonding 53 minLecture18.1
-
Ionic Bonding, covalent bonding 50 minLecture18.2
-
Ionic Character in Covalent Bonding, Electronegativity 34 minLecture18.3
-
Dipole Moment 42 minLecture18.4
-
Fajan’s Rule 34 minLecture18.5
-
Model for Covalent Compound, V.B.T. – Lewis St. Model 56 minLecture18.6
-
Lewis Structure Model 45 minLecture18.7
-
Formal Charge 46 minLecture18.8
-
Formal Charge Rule 44 minLecture18.9
-
Resonance 43 minLecture18.10
-
Merits & Demerits of Lewis St. Model 44 minLecture18.11
-
Drawing Lewis St. 30 minLecture18.12
-
VSEPR 1 49 minLecture18.13
-
VSEPR 2 51 minLecture18.14
-
VSEPR 3 51 minLecture18.15
-
VSEPR 4 33 minLecture18.16
-
BackBonding 38 minLecture18.17
-
Bond Angle determination 47 minLecture18.18
-
Concept of Hybridisation 44 minLecture18.19
-
Sp3, Sp2 Hybridisation 44 minLecture18.20
-
SP hybridisation, Relative study of SP, SP2, SP3 Hybridisation 46 minLecture18.21
-
Hybridsation involving D-orbitals 39 minLecture18.22
-
Hybridsation with D-orbitals, Limitation of Hybridisation 41 minLecture18.23
-
Calculation of Hybridisation of Central Atom, Problems 43 minLecture18.24
-
Merits & demerits of VBT, Introduction to MOT 33 minLecture18.25
-
MO formation, Bond Order 43 minLecture18.26
-
MO with P-orbitals, B2, Magnetic Character 43 minLecture18.27
-
MO of Diatomic Species, Hetroatomic Species 51 minLecture18.28
-
Secondary Bondings 39 minLecture18.29
-
H Bonding 37 minLecture18.30
-
Metallic Bonding 52 minLecture18.31
-
Chapter Notes – Chemical BondingLecture18.32
-
-
19. Periodic Table
10-
Development of P.T. 43 minLecture19.1
-
Mandeelev P.T. & Mosley, Modern P.T. 43 minLecture19.2
-
Modern P.T. & Periodic Properties 27 minLecture19.3
-
Atomic Volume & Radius 49 minLecture19.4
-
Atomic Radius, Ionisation Energy 28 minLecture19.5
-
Ionisation Energy 48 minLecture19.6
-
Electron Affinity, Hydration Energy 52 minLecture19.7
-
Electronegativity, Lattice Energy 46 minLecture19.8
-
Oxidising & Reducing Power, Nature of oxides 38 minLecture19.9
-
M.P. & B.P., Density, Bond Energy, Diagonal relationship, Inert Pair Effect 25 minLecture19.10
-
-
20. Metallurgy
7-
Introduction, Concentration of ore 49 minLecture20.1
-
Roasting, Calcination, smelting 41 minLecture20.2
-
Refining of metal 29 minLecture20.3
-
Pyrometallurgy, electrometallurgy, Hydrometallurgy 32 minLecture20.4
-
Ellingham Diagram 43 minLecture20.5
-
Extraction of Cu & Fe 22 minLecture20.6
-
Extraction of Al & Zn 26 minLecture20.7
-
-
21. Hydrogen and its Compounds
7-
preparation, properties & Type of Hydrogen 57 minLecture21.1
-
Compounds of Hydrogen, Hydrides, Water, Hydrates 56 minLecture21.2
-
Hardness of Water, H2O2 56 minLecture21.3
-
Problems 48 minLecture21.4
-
Problems 28 minLecture21.5
-
Chapter Notes – Hydrogen and its CompoundsLecture21.6
-
NCERT Solutions – HydrogenLecture21.7
-
-
22. S block metals
8-
IA 1 – elemental Properties of Alkali metals& its Compounds 57 minLecture22.1
-
IA 2 – Na & its compounds 01 hourLecture22.2
-
IA 3 – Na & its Compounds, Use of Na & K 27 minLecture22.3
-
IIA 1 – Elemental Properties 41 minLecture22.4
-
IIA 2 – Compounds of IIA Metals 53 minLecture22.5
-
IIA 3 – Compounds of Ca 48 minLecture22.6
-
Chapter Notes – S block metalsLecture22.7
-
NCERT Solutions – S block metalsLecture22.8
-
-
23. p block elements
8-
Introduction to P – Block & IIIA – elemental properties 51 minLecture23.1
-
IIIA – General properties of compounds & B-compounds 40 minLecture23.2
-
IIIA – Boron compounds, Use of B and Al 35 minLecture23.3
-
IVA – Elemental Properties of C family 46 minLecture23.4
-
IVA – Allotropes of C & compounds of C 01 hourLecture23.5
-
IVA – Compounds of Si 48 minLecture23.6
-
Chapter Notes – p block elementsLecture23.7
-
NCERT Solutions – p block elementsLecture23.8
-
Chapter Notes – p block elements
Elements in which the last electron enters in the any one of the three p- orbital of their outermost shells – p-block elements
• Gen. electronic configuration of outer shell is ns2np1-6 The inner core of e-config.may differ which greatly influences their physical & to some extent chemical properties.
• The block of elements in the periodic table consisting of the main groups :
| • Group13 | (B to Tl) |
| • Group14 | (C to Pb) |
| • Group15 | (N to Bi) |
| • Group 16 | (O to Po) |
| • Group17 | (F to At) |
| • Group18 | (He to Rn) |
(1) Members at the top and on the right of the p-block are nonmetals (C, N, P, O, F, S, Cl, Br, I, At).
(2) Those on the left and at the bottom are metals (Al, Ga, In,Tl, Sn, Pb, Sb Bi, Po).
(3) Between the two, from the top left to bottom right, lie an ill-defined group of metalloid elements (B, Si, Ge, As, Te)
GROUP 13 : The boron group
• Outer Electronic Configuration:-ns2np1
• group members: boron (B), aluminum (Al), gallium (Ga), indium (In) & thallium (Tl) . All, except boron, are metals.
• Boron show diagonal relationship with Silicon; both are semiconductors metalloids & forms covalent compounds.
• Boron compounds are electron deficient, they are lack of an octet of electrons about the B atom .
• diborane B2H6 is simplest boron hydride
• Structure: three-center two-electron: the H atoms are simultaneously bonded to two B atoms the B-H bridging bond lengths are greater than B-H terminal.
• – Boron oxide is acidic (it reacts readily with water to form boric acid)
• aluminium compounds:aluminium oxide is amphoteric
• aluminum halides, e.g., AlCl3 is dimer, an important catalyst in organic chemistry have anincomplete octet, acts as Lewic acid by accepting lone pairs from Lewic bases, forming adduct
• aluminum hydride, e.g., LiAlH4, a reducing agent
• Atomic Properties – Electronic Configurations
| Element | Symbol | Atomic No. |
Electronic Configuration |
Abundance in Earth’s Crest (in ppm) |
| Boron | B | 5 | [He]2s2 2p1 | 8 |
| Aluminium | Al | 13 | [Ne]3s2 3p1 | 81,300 |
| Galium | Ga | 31 | [Ar]3d104s2 4p1 | 15 |
| Indium | In | 49 | [Kr] 4d105s2 5p1 | 1 |
| Thallium | Tl | 81 | [Xe] 5d106s2 6p1 | 0.3 |
Atomic and ionic radii
• The atomic and ionic radii of group 13 elements are compared to corresponding elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge.
• In other words, effective nuclear charge increases and thus, size decreases. Therefore, the elements of this group have smaller size than the corresponding elements of second group.
• On moving down the group both atomic and ionic radii are expected to increase due to the addition of new shells. However, the observed atomic radius of Al (143 pm) is slightly more than that of Ga (l35 pm).
Ionization energies
The first ionization energies of group 13 elements are less than the corresponding members of the alkaline earths.
The sharp decrease in I.E. from B to Al is due to increase in size. In case of Ga, there are ten d-electrons in its inner electronic configuration.
The very high value of 3rd I. E. of thallium indicates that +3 O.N. state is not stable, rather +1 is more stable for thallium .
Electropositive (or metallic) character
the elements of group 13 are less electropositive as compared to elements of group 2. On moving down the group the electropositive (metallic) character increases because ionization energy decreases. For e.g., Boron is a non-metal white the other elements are typical metals.
Oxidation states
The common oxidation states of group 13 elements are +3 and + l .The stability of the + 1 oxidation state increases in the sequence Al <Ga< In <Tl, Due to Inert pair effect.
| Element | B | Al | Ga | In | Tl |
| Oxidation state | +3 | +3 | +3, +1 | +3, +1 | +3, +1 |
Chemical reactivity of Gr.13 Elements
All elements in their compounds exhibit the oxidation state of + 3 and +1. Hydrides
• None of the group 13 elements reacts directly with hydrogen. However, a no.
of hydrides of these elements have been prepared by indirect methods. The boron hydrides are called boranes& classified in two series: (a) BnHn+4 called nidoboranes (b) BnHn+6 called arachnoboranes
• INUDUSTRIAL PREPERATION :-
2BF3(g) + 6LiH(s) → B2H6(g) + 6LiF(s)
• Laboratory method:
(i) By the reaction of iodine with sodium borohydride in a high boiling solvent. 2NaBH4 + I2 → B2H6 + 2NaI + H2
(ii) By reduction of BCl3 with LiAlH4 4BCl3 + 3LiAlH4 → 2 B2H6 + 3AlCl3 + 3 LiCl
Structure of Diborane, B2H6
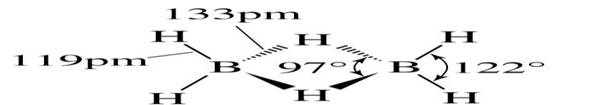
Some important characteristics of boranes:
i) Lower boranes are colourless gases while higher boranes are volatile liquids or solids.
ii) They undergo spontaneous combustion in air due to strong affinity of boron for oxygen. B 2H6 + 3O2 → B2O3 + 3H2O + Heat
iii) Boranes react with alkali metal hydrides in diethyl ether to form borohydride complexes. B2H6 + 2MH →2M+[BH4]- (M= Li or Na) Metal borohydride
(iv) Diborane reacts with ammonia to give borazine at 450 K. B2H6 + 6NH3 → 3B3N3H6 + 12H2
• Borazine has a cyclic structure similar to benzene and thus is called inorganic benzene
• The other elements of this group form only a few stable hydrides. The thermal stability decreases as we move down the group.
• AlH3 is a colourless solid polymerized via Al – H – Al bridging units. These hydrides are weak Lewis acids and readily form adducts with strong Lewis base (B:) to give compounds of the type MH3 (M = Al or Ga). They also form complex-tetrahydrido anions, [MH4]-. The most important tetrahydrido compound is Li[AlH4]
![tetrahydrido compound is Li[AlH4]](https://ncerthelp.com/ncertimages/class%2011/chemistry/CH%2011/tetrahydrido%20compound%20is%20%20Li[AlH4]jpg.jpg)
OXIDES & HYDROXIDES
• M2O 3 & M(OH)3
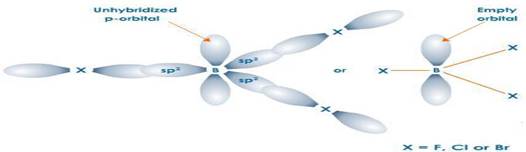
HALIDES: Structure of boron trihalides
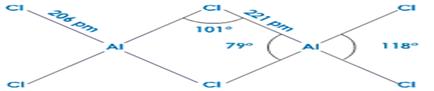
Dimeric structure of aluminium chloride
– Boron halides do not form dimers because the size of boron is so small that it is unable to coordinate four large-sized halide ions.
• Anomalous properties of boron
1. Boron is a non-metal & bad conductor of electricity whereas aluminium is a metal& good conductor. B is hard but Al is a soft metal.
2. Boron exists in two forms-crystalline and amorphous. But Al does not exist in different forms.
3. The melting and boiling point of boron are much higher than that of Al .
4. Boron forms only covalent compounds whereas Al forms even some ionic compounds.
5. The hydroxides and oxides of boron are acidic in nature whereas those of aluminium are amphoteric.
6. The trihalides of boron exist as monomers. On the other hand, aluminium halides exist as dimers .
7. The hydrides of boron are quite stable while those of aluminium are unstable
• Boron and silicon exhibit the typical properties of non-metals. These do not form cations. Both exist in amorphous as well as crystalline forms.
• Boron oxide (B2O3) and silic a (SiO2) both are acidic and dissolve in alkali solutions to form borates and silicates respectively. B2O3 + 6NaOH → 2Na2BO3 + 3H2O SiO2 + 2NaOH → Na2SiO3 + H2O
• The chlorides of both B and Si get hydrolyzed by water to boric acid and silicic acid respectively. BCl3 + 3H2O →H3BO3 + 3HCl SiCl4 + 3H2O → H2SiO3 + 4HCl
The hydrides of Boron and Silicon are quite stable. Numerous volatile hydrides are also known which catch fire on exposure to air and are easily hydrolyzed. Both elements are semiconductors.
Behavior in Aqueous Solutions
1 Al, Ga, In and Tl exhibit a well-defined aqueous chemistry in their tripositive states. Species like [M(OH)4]–, [M(H2O)2(OH)4]–, [M(OH2)6]3+ for M = Al, Ga, In, exist in aqueous solution.
2. Al, Ga. In and T1 ions exist as octahedral aqua ions, [M(OH2)6]3 + in aqueous solution and many salts like halides, sulphates, nitrates and perchlorates exist as hydrates.
3. Aluminiumsulphate forms double salts – called alum, having the general formula M2SO4. Al2(SO4)3.12H2O, where M=Na+ or K+. USES OF BORON & ALUMINIUM
• Aluminium is used extensively in industry and everyday life. It forms many useful alloys with Cu. Mn, Mg, Si and Zn. Hence, aluminium and its alloys find use in packaging, utensil making, construction, aerospace and other transportation industries. It is used as a conductor for transmission of electricity. Aluminium is alsoused in the alumino-thermite process for production of chromium and manganese from their ores.
Group 14 Elements:-The Carbon Family
Group 14 includes carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb). General electronic configuration of carbon family is ns2np2. Covalent radius:-Covalent radius expected to increase from Cto Si, From Si to Pb small increase is found.
Ionization Enthalpy:-The first ionization enthalpies of group 14 elements are higher than those of the corresponding group 13 elements. Electronegativity:-Group 14 elements are smaller in size as compared to group 13 elements that’s why this group elements are slightly more electronegative than group13
Chemical properties:-
Carbon and silicon mostly show +4 oxidation state. Germanium forms stable compounds in +4 state and only few compounds in +2 state.
Tin forms compounds in both oxidation states. Lead compounds in +2 state are stable and in +4 state are strong oxidizing agents.
Exception:-Pb4 and SnF4 are ionic in nature.
Except CCl4 other tetrachlorides are easily hydrolysed by water.
Since carbon does not have d-orbitals and hence cannot expand its coordination number beyond 4
CCl4 +H2O No Reaction
SiCl4+4H2O Si(OH)4+4HCl Silicic acid
Allotropes of Carbon:-The three types of allotropes are –
1-Diamond
2-Graphite
3-Fullerence
Diamond :-
In diamond each carbon atom undergas SP3hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms.
Graphite :-
In graphite, carbon is SP2-hyberdized graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Graphite conducts electricity along the sheet.It is very soft and Slippery FullerenceFullerence was discovered collectively by three scientists namely R.E Smalley,R.F Curl and H.W Kroto SOME Important Compounds Of Carbon and Silicon
Carbon monoxide :-
It I prepared by direct oxdisation of C in limited supply of oxygen. 2C+O2(g) → 2CO(g Commercially it is prepared by the passage of steam over hot coke
Carbon Dioxide :-
It is prepared by complete combustion of carbon and carbon fuels in excess of air.C(s) +O2(g) → CO2(g)
Laboratory method:-
In laboratory it is prepared by the treatment of dilHCl on CaCO3 CaCO3(s) +2HCl(aq) → CaCl2(aq) +CO2>/(g)+H2O(l) Silicon
Dioxide :-
Silicon dioxide is a COVALENT THREE DIMENSIONAL NETWORK SOLID.
Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Silicones:-Silicones are the synthetic organo-siliconpolymers having general formulae (R2SiO)n in which R = alkyl (methyl,ethyl or phenyl) Silicates:-Silicates are exist in nature in the form of feldspar, zeolites,mica and asbestos etc. The basic structure of silicates is SiO44
Zeolite s:
-Zeolites is aalumino-silicate of metal. Metal cations participating in formationof Zeolite are use usually Na+,K+,or Ca2+. Zeolites are used to remove permanent hardness of water.
